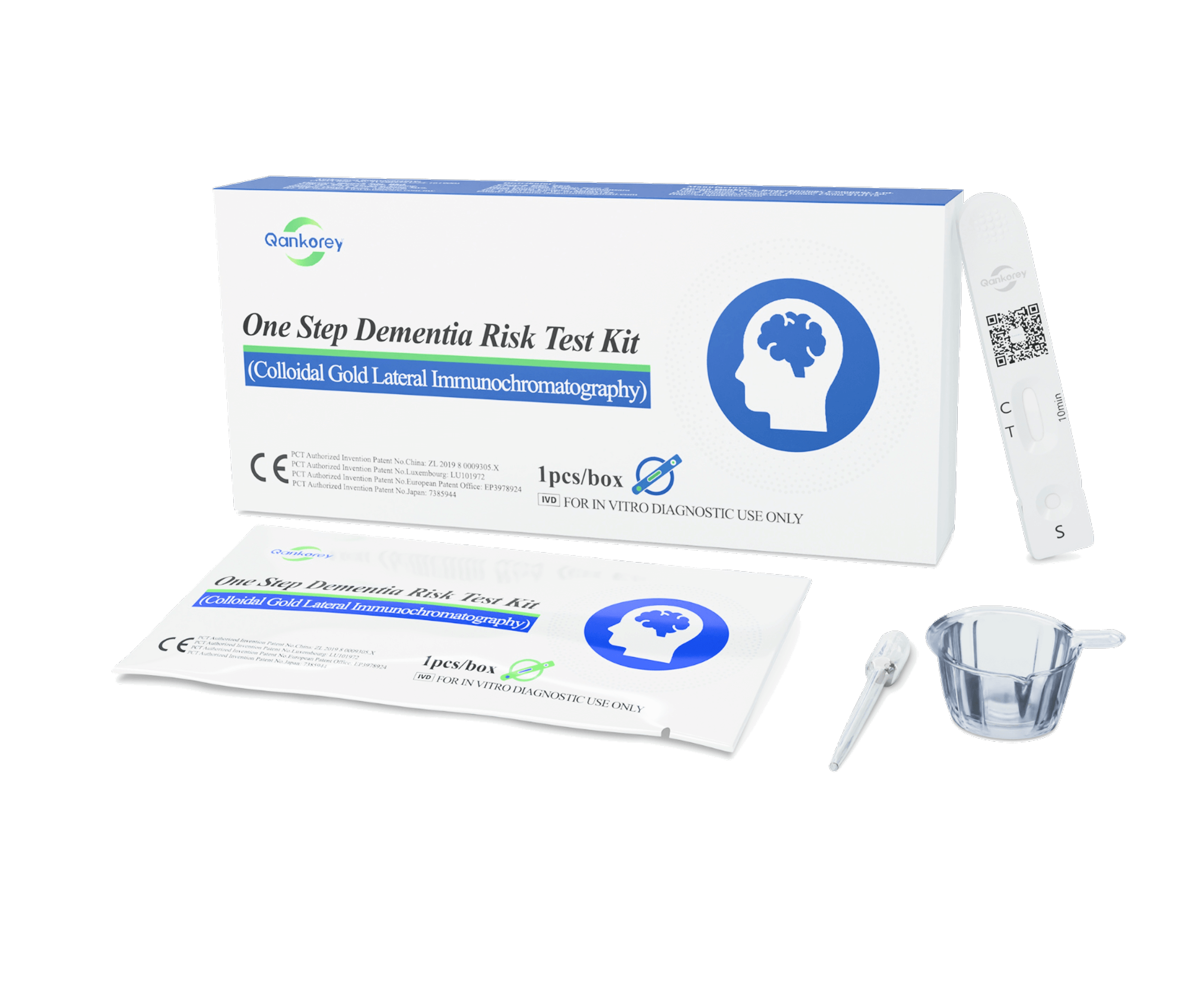
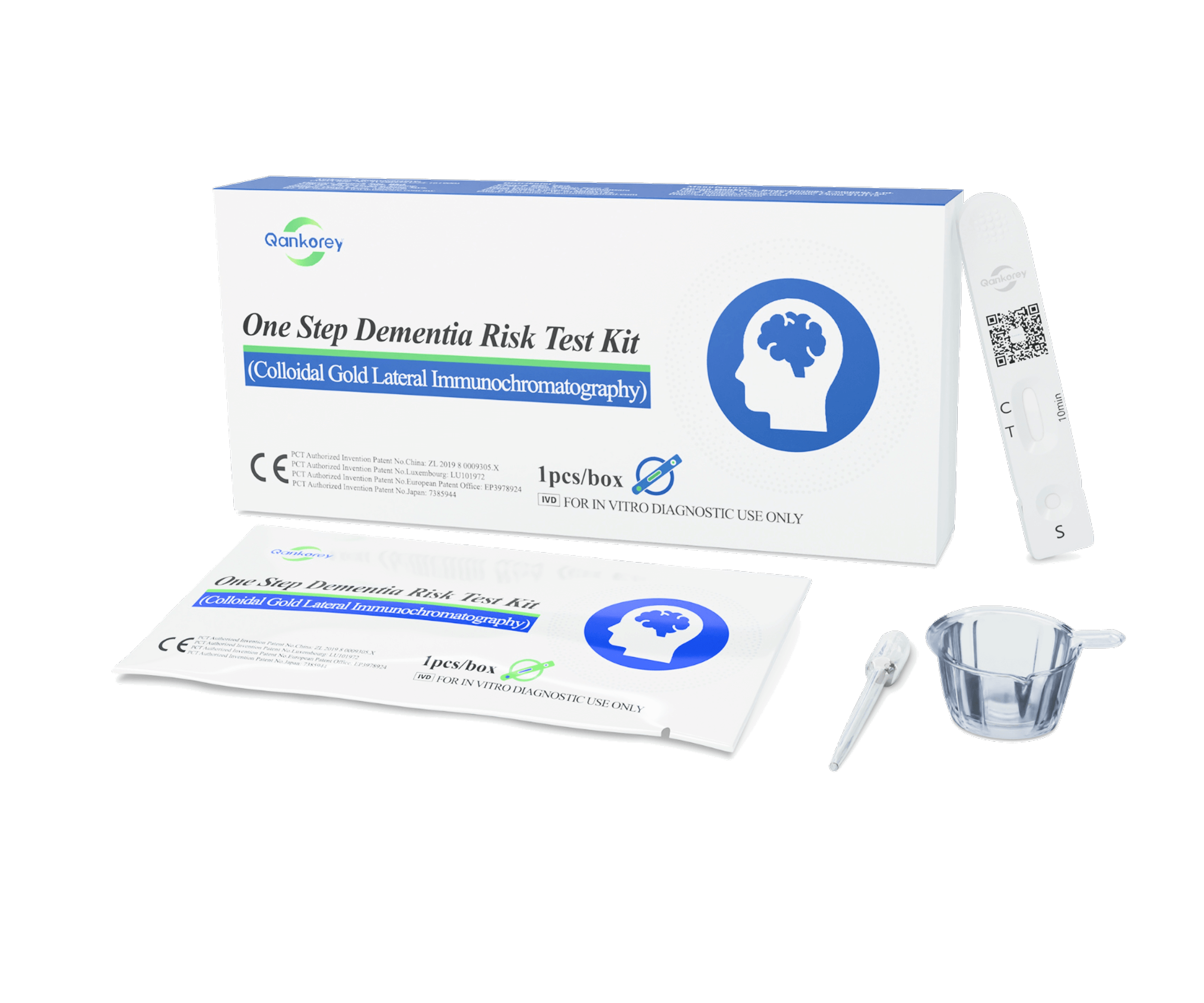
– Sample collection:
+ This kit is designed for collecting human urine samples.
- The sample must be collected in a clean, dry container. Urine can be collected at any time of the day. Fresh urine does not require special processing. It is recommended to drink 200–300 mL of water 30–60 minutes before sample collection.
- The sample should be used as soon as possible after collection and should not be stored at room temperature for more than two hours. If refrigerated, it may be stored at 2–8 °C for up to six hours before testing. Refrigerated samples should be brought to room temperature and mixed thoroughly before testing.
- Do not use frozen and thawed samples unless for research purposes.
– Test procedure:
This kit can be used by consumers or healthcare professionals in clinical facilities, pathology laboratories, and research institutes. Before testing, read the “Instructions for Use” and allow the test kit and other necessary items to reach room temperature. Do not open the foil pouch until ready to perform the test, and use the kit within 1 hour after opening. If the ambient temperature exceeds 30 °C or humidity exceeds 75%, the test should be performed immediately after opening.
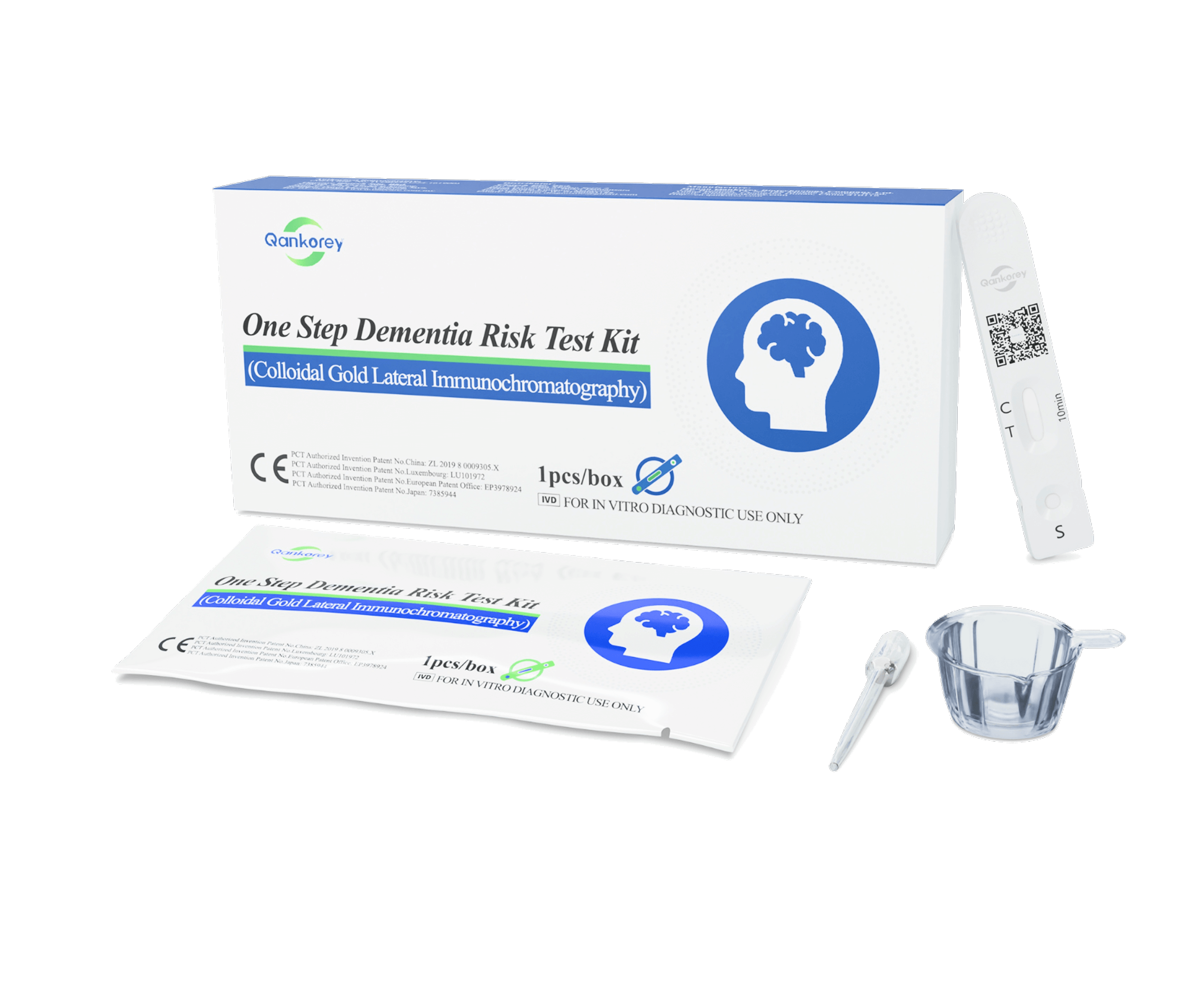
- Tear open the foil pouch and remove the test cassette.
- Using the provided dropper, collect approximately 100 µL (~4 drops) of fresh urine and slowly add the drops into the sample well of the cassette.
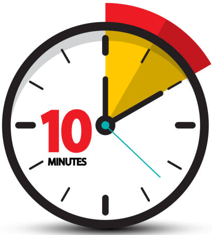
- Place the cassette on a flat surface for 10 minutes before reading the results. Results are unreliable after 15 minutes.
– Interpretation of results:
+ Positive: Two reddish-purple lines appear—one in the test region (T) and one in the control region (C).
+ Negative: One reddish-purple line appears in the control region (C) with no line in the test region (T).
+ Invalid: No reddish-purple line appears in the control region (C), indicating an operational error or a damaged test cassette.
– Storage conditions and shelf life:
+ Store at 4–25 °C. Shelf life: 18 months.
+ For single use only.
+ Store in a dry place, away from light, high temperatures, and do not freeze. Under extreme weather conditions, protective measures should be taken to prevent exposure to high temperatures or freeze–thaw cycles.